PRODUCT
Rapid Polymyxin™ Pseudomonas
Rapid Detection of Resistance to Polymyxins The Rapid Polymyxin NP test has been designed for the detection of Pseudomonas aeruginosa susceptibility and resistance to polymyxins (Colistin, and polymyxin B).
- Fast results in 3-4 hours
- Reliable approach, regardless of the molecular mechanism of resistance (MCR1 and others)
- Easy to perform, highly specific and sensitive
- Excellent correlation with the reference broth microdilution method in 24h
Catalogue number(s):
23001
Product Description : Rapid Polymyxin PSEUDOMONAS
Number of Tests : 10 tests

Image Gallery
Benefits
The emergence and global spread of carbapenemase-producing Pseudomonas aeruginosa are of great concern to health services worldwide. Because these bacteria usually remain susceptible to polymyxins, interest in this old class of antibiotics has been recently renewed. However, the increasing use of colistin explains why aeruginosa strains resistant to colistin are increasingly reported worldwide. Currently available polymyxins susceptibility methods are fastidious, time-consuming (24 hours) and some methods are not reliable. They are poorly adapted to the clinical need and to the prevention of the dissemination of those multidrug resistant isolates. ELITechGroup Microbiology releases its new Rapid Polymyxin NP test proposed as a rapid, reliable and cost-effective test to detect polymyxins (polymyxin E or Colistin, polymyxin B) resistant Pseudomonas. This liquid method relies on the rapid and easy visualization of colorimetric reactions. The Rapid Polymyxin Pseudomonas test is easy-to-perform, very sensitive and specific. It can detect in only 3-4 hours polymyxin-resistant and –susceptible P. aeruginosa isolates , regardless of the molecular mechanism of polymyxins resistance. This test offers the possibility of detecting polymyxins resistance from bacterial cultures before any antimicrobial drug susceptibility testing results are obtained. Results are obtained at least 16 hours sooner with this test than with the reference broth microdilution method. It is close in performance to the reference dilution technique but much less cumbersome and is not based on diffusion of large polymyxin molecules in agar, which therefore prevents false susceptibility results.
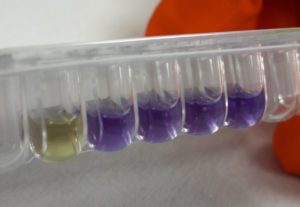
“
REAGENTS AND MATERIAL
| Description | Quantity |
|---|---|
| RP NaCl: Vial of 3mL of liquid medium containing 0.85 g/L of NaCl for the preparation of the inoculum | 12 |
| RP Medium Pseudomonas: Vial of 1.5 mL of culture medium for Pseudomonas aeruginosa, based on a nutrient broth enriched with a growth factor, a sugar and Bromocresol purple (a pH indicator) | 10 |
| RP PSEUDOMONAS tray: Tray containing a negative control C- well, two Test wells containing colistin at concentrations of 2, 4 and 8 mg/L and a bacterial growth control well C +. Tray packed in an aluminum sachet with an integrated dessiccant. | 10 |
| RP TC (Turbidity Control): Vial of 3 mL of barium sulphate solution for turbidity control | 1 |
| CLOSING SYSTEM: Protective translucent plastic tray lid for the inoculated tray | 10 |
STABILITY AND STORAGE
- All of the provided reagents are ready-to-use. The kit and the reagents when stored at 2-8°C in their original state are stable until the expiry date indicated on the box.
- The RP Medium Pseudomonas and RP NaCl are single use reagents.
- If the RP TC vial is used as a control for inoculum calibration of isolated colonies, it has to be kept until the last RP Medium Pseudomonas vial of the kit has been inoculated.
- During storage, the RP TC reagent must be protected from the light and maintained at 2-8°C.
PERFORMANCE
A multicentric performance evaluation was performed in three laboratories with bacterial isolates from different clinical samples:
- The National Research Institute (CNR) of Antibiotic Resistance, Bacteriology Laboratory, CHRU, Besançon, France,
- Emerging Antibiotic Resistance Unit (Medical and Molecular Microbiology, Department of Medicine, University of Fribourg, Switzerland) – Pr. P. Nordmann
- Research and Development Laboratory at ELITEch Microbio, Signes, France
The minimum inhibitory concentrations (MIC) of the tested isolates were determined according to the liquid micro-dilution reference method (Mueller-Hinton cation adjusted broth), according to the guidelines of the Clinical Laboratory Standard Institute (CLSI) (1,2). The percentage of clinical concordance of the RAPID POLYMYXIN PSEUDOMONAS test is 90.5% compared to the MIC method in liquid medium. The sensitivity of the test is 89.7% and the specificity is 90.8% .
A total of 148 strains were tested. The distribution panel of the tested strains is as follows:
| Susceptible strains N=119 | #colspan# | #colspan# | #colspan# | #colspan# | Resistant strains N=29 | #colspan# | #colspan# | #colspan# | #colspan# | #colspan# | #colspan# | #colspan# | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC ref. (mg/L) | 0.12 | <0.25 | 0.5 | 1 | 2 | 4 | 8 | >8 | 16 | 32 | 64 | >128 | TOTAL |
| Number of strains | 1 | 6 | 18 | 75 | 19 | 3 | 7 | 6 | 1 | 3 | 7 | 2 | 148 |
MATERIAL REQUIRED BUT NOT PROVIDED
- Waste container for contaminated waste
- Densitometer
- Pipettes and tips
- Certified incubator at +36°C+/-2°C
“
Let us help you
For general inquiries, please use the links to the right. Click Contact to complete a brief online form, or click Support for general phone and email information. Someone will be in touch with you soon.

