PRODUCT
MYCOFAST® RevolutioN ATB+ (DIRECT)
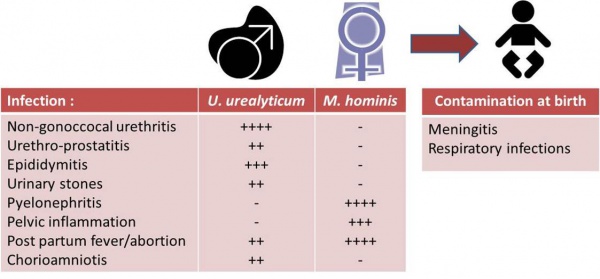
Detection, enumeration, identification and complete antimicrobial susceptibility testing of urogenital mycoplasma: DIRECT METHOD
NEW GENERATION: “Expanding the scope of treatment options!”
- Extended panel of antibiotics tested (x11) !
- 100% compliance with worldwide recommendations !
The MYCOFAST® RevolutioN ATB+ is an accurate, cost-effective, easy-to-use colorimetric assay designed for the detection, enumeration, identification and antibiotic susceptibility determination of Ureaplasma urealyticum (U.u.) and Mycoplasma hominis (M.h.) from urogenital specimens. The MYCOFAST® RevolutioN ATB+ enables labs to determine susceptibility to a panel of new antibiotics, helping physicians comply with the latest therapeutic guidelines from CLSI (Clinical Laboratories Standards Institute). The addition of the MYCOFAST® RevolutioN ATB+ expands ELITechGroup’s family of mycoplasma test solutions, offering the most extensive antibiotic susceptibility determination at key clinical end-points.
- Extended panel of antibiotics tested, in full compliance with worldwide recommendations
- Complete diagnosis in an all-in-1 product
- The right pathologic threshold tested for each specimen type
- Fast results for clinicians
- Visual, colorimetric reading
Featuring ready-to-use reagents and an “all liquid” method, MYCOFAST® RevolutioN ATB+ identifies the growth of U.u. and M.h. after 24 hours of incubation. Our exclusive growth activator, the M.h. Supplement, guarantees fast and accurate results. Moreover, MYCOFAST® RevolutioN ATB+ enables the enumeration of U.u. > 103 CCU/mL which is compliant with pathological thresholds recognized for sperm and urine samples. Finally, the UMMt RevolutioN & UMMt RevolutioN AMIES transport media enable optimized sample management by preserving specimen integrity up to 72 hours.
Catalogue number(s):
| Reference | Name | Quantity |
| 00070 | MYCOFAST® RevolutioN ATB+ | 25 tests |

Image Gallery
Benefits
Intended use
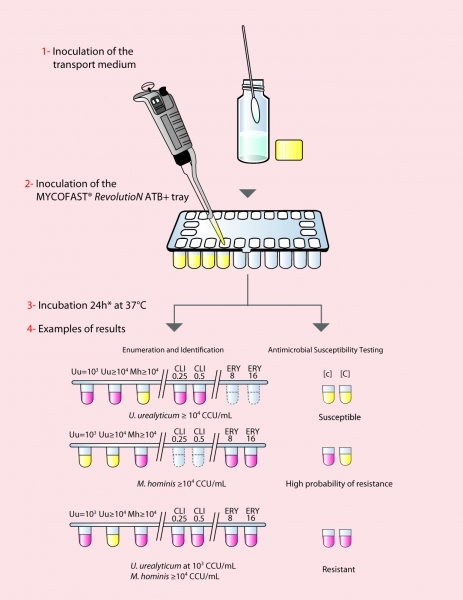
Principle
MYCOFAST® RevolutioN ATB+ identifies U.u. and M.h. growth after 24 hours of incubation in a liquid medium. During growth, U.u. and M.h. metabolize urea and arginine respectively resulting in a color change of the medium, which contains phenol red indicator, from yellow-orange to red. Mycoplasma growth thus viewed enables:
- Enumeration of mycoplasma based on the rate of urea or arginine hydrolysis, which is proportional to the number of germs contained in the sample. U.u: > 103 and 104 UCC/ml M.h: > 104 UCC/ml
- Identification secured by checking U.u/M.h natural resistance to 2 antibiotics (Clindamycin and Erythromycin)
- U.u and M.h susceptibility testing to 6 antibiotics compliant with worldwide recommendations: Levofloxacin (LVX) 1-2-4 μg/mL , Moxifloxacin (MXF) 0.25-0.5-2-4 μg/mL , Erythromycin (E) 8-16 μg/mL, Clindamycin (CM) 0.25-0.5 μg/mL Tetracyclin (TE) 1-2-4-8 μg/mL, and Telithromycin 4μg/mL
- U.u and M.h susceptibility testing to 5 other antibiotics: Minocycline (MIN) 2µg/ml, Ofloxacin (OFX) 1µg/ml, Josamycin (JOS) 2µg/ml, Pristinamycin (PRI) 2µg/ml and Roxithromycin (ROX) 1µg/ml
Easy-to-read and easy-to-interpret results
Reagents and material
- UMMt RevolutioN: Vial of 3 mL mycoplasma broth with antimicrobial agents and preservative solution. pH: 6.0 ± 0.1 25 Quantity: 25
- MYCOFAST® RevolutioN ATB+: Tray of 24 wells packed in an aluminium sachet with an integrated desiccant Quantity: 25
- Closing system: Protective translucent plastic tray lid Quantity: 25
Material required but not provided
- Sample collecting material (Swabs, cytobrushes, sterile containers for liquid samples)
- MYCOPLASMA Stabilizer (REF 00064)
- Pipettes and tips
- Waste container for contaminated waste
- Mineral oil
- Incubator at 37 ± 1 °C
Stability and storage
- All the reagents are ready-to-use. The vials may be stored at 2-8 °C, in their original packaging until the expiry date shown on the kit.
- The UMMt medium may be stored temporarily (3 months) at room temperature but is more stable at 2-8 °C.
- Do not freeze the reagents contained in the kit.
Let us help you
For general inquiries, please use the links to the right. Click Contact to complete a brief online form, or click Support for general phone and email information. Someone will be in touch with you soon.